For patients who can’t quit smoking there are several reduced-harm alternatives.
None of these alternative nicotine products are safe. They should not be used except as substitutes by patients who can’t quit.
Below is the final report from Dr. Cheryl K. Olson, MPH, Sc.D., whose work was funded with a grant from the Foundation for a Smoke-Free World, Inc. a US nonprofit 501(c)(3) private foundation. The contents, selection, and presentation of facts, as well as any opinions expressed herein, are the sole responsibility of Dr. Olson and of the Physicians Research Institute and under no circumstances shall be regarded as reflecting the positions of the Foundation for a Smoke-Free World, Inc.
Joseph A. Schwartz, III
President, Physicians Research Institute
There is an urgent need to get patients to quit combustible tobacco. This is especially true for longtime smokers, at risk of immediate harm. Quit rates for Americans over 65 have been stagnant since the turn of the century.[1]
What can I do for patients who can’t, or won’t, quit smoking?
Physicians around the world report feeling frustrated by this situation.[2] Some patients may have tried and failed with cessation medications: nicotine replacement therapies (NRT), varenicline, or buproprion.[3] Others reject patches and pills as substitutes for their cigarette habit.
Fortunately, there are now other options to consider, that can deliver nicotine in much less dangerous ways than cigarettes. You’ve heard of some. Others may be completely new to you. These alternative nicotine products are made and regulated outside of the familiar medical pathways. Product types include modern oral tobacco, e-cigarettes, and heated tobacco products.
Pioneering nicotine addiction researcher Karl Fagerstrom notes that countries with higher uptake of these alternative products have lower smoking rates than neighboring nations. He says such products could be “the final nail in the smoking coffin”.[4]
Are these alternative nicotine products approved by the FDA?
These are not FDA-approved quit-smoking methods. However, they are regulated by the Food and Drug Administration. Based on research submitted to FDA’s Center for Tobacco Products, some product brands have received marketing authorization. That means they are sold legally. A few have earned the right to be called “modified risk tobacco products.”
What is a “modified risk tobacco product”?
This concept comes from Section 911 of the 2009 Family Smoking Prevention and Tobacco Control Act. That Act is the basis of our current U.S. tobacco product regulatory regime. It says a “modified risk tobacco product” is one “sold or distributed for use to reduce harm or the risk of tobacco-related disease.” Companies must invest extra time and resources to get MRTP status. Along with the usual scientific evidence for reduced health risks, studies of consumer perceptions and real-world product use are required.
As part of this process, a company seeks permission to use product-specific reduced-harm wording on their labels. Without MRTP authorization, a nicotine product maker can’t say anything on its labeling, advertising or other media “that would be reasonably expected to result in consumers believing that the tobacco product or its smoke may present a lower risk of disease or is less harmful.” Or, that their product or its smoke/vapor has fewer bad things in it. The 2009 Act also prohibits the use of weasel words like “light,” “mild,” or “low.”
So far, only three alternative products (as well as a low-nicotine cigarette) have received modified risk tobacco product designations: General Snus, IQOS, and Copenhagen Snuff.
Note that the Center for Tobacco Products does not use the usual FDA “safe and effective” standard. Instead, its reviewers scrutinize whether allowing the sale of a particular product would overall—balancing the risks and benefits to tobacco users and to non-users—be a plus for public health.
Here is a link to FDA’s current list of nicotine products that have marketing authorizations. Many other products are under FDA review and are legally on the market in the meantime. Some companies flout the FDA and sell their products illegally.
What do I tell patients who ask about nicotine alternatives?
Surveys show that patients are increasingly asking their doctors about these products.[5] [6] You may have run across them yourself at convenience stores or gas stations, or seen ads or online videos. Making any recommendations in this Wild West of proliferating reduced-harm options can feel daunting.[7]
Below is a roundup of three common nicotine alternatives: smokeless tobacco, electronic cigarettes, and heated tobacco products. What do they look like? How are they used? What do we know about their risks? And what evidence do we have that they might attract patients away from cigarettes?
Smokeless (Oral) Tobacco Products
What are smokeless tobacco products?
As a recent review[8] notes, “Extraordinarily diverse types of smokeless tobacco products with varying carcinogenic properties are consumed in the world.”
Options sold in the U.S. include:
- Chewing tobacco.
- Moist and dry snuff.
- Snus
- Tobacco-free pouches.
- Nicotine lozenges, gums, and toothpicks.
Moist snuff is the top-selling U.S. smokeless product. Snus, tobacco-free pouches and nicotine lozenges are gaining popularity.[9]
What is moist snuff?
Using this finely ground tobacco product (known as “dipping”) involves tucking a pinch between the lip and gum. Users may spit the excess saliva generated. Popular brands of moist snuff include Copenhagen, Grizzly and Skoal.

How risky is using moist snuff compared to smoking?
Relative risks are hard to summarize due to issues with research. Most studies are decades old, and many didn’t separate out the effects of smoking and smokeless tobacco.[10]
One recent analysis of U.S. federal longitudinal study data[11] compared people who used only snuff and/or chewing tobacco to people who had never smoked or used any other kind of tobacco. The risk of death from all cancers combined was not higher for smokeless tobacco users compared to tobacco non-users. Moist snuff users did have a somewhat higher risk of death from heart disease, similar to the extra heart risk from secondhand cigarette smoke exposure.
In 2023, the FDA granted Copenhagen Classic Snuff the right to be marketed as a modified risk tobacco product. FDA says research support this claim: “Switching completely to this product from cigarettes reduces risk of lung cancer.”
What is snus?
Snus is basically moist snuff in ready-to-use small pouches that resemble small tea bags. The pouch is tucked between the lip and gum; there is no spitting. Popular brands include General Snus and Camel Snus.

How risky is using snus compared to smoking?
Well, Sweden has the lowest rate of smoking and of male tobacco-related mortality in the European Union. Widespread use of snus gets the credit.[12] (Snus use is much more common among men.) A large Swedish longitudinal study found that when men who smoked took up snus, 76% stopped all cigarette use.
Eight General brand snus products from Swedish Match were the first ever to receive the FDA’s modified risk tobacco product designation. FDA says this claim is supported by research: “Using General Snus instead of cigarettes puts you at a lower risk of mouth cancer, heart disease, lung cancer, stroke, emphysema, and chronic bronchitis.”
What is a nicotine pouch?
Tobacco-free nicotine pouches are small and white, made with plant fibers, various flavors and sweeteners, and nicotine. They’re used like snus; you tuck the pouch between your cheek and gum. Popular brands include Zyn, On! and Velo.

How risky are nicotine pouches compared to smoking?
Because this product type is relatively new, there’s little independent research. The toxicant profile is similar to nicotine replacement therapies, so pouches might be close to NRT in terms of risk.[13]
In a Rutgers University national survey of physicians5, about ten percent said patients had asked about tobacco-free nicotine pouches. A 2021 survey of adults who smoked[14] found that 29% were aware of nicotine pouches, and 5.6% had tried them. More smokers were willing to try pouches compared to other kinds of smokeless tobacco.
What does “tobacco-free” mean?
This has two meanings in research literature and in advertising:
1) the product has no tobacco leaf in it.
2) the product contains nicotine made in a lab rather than from a tobacco plant.[15] Products made with synthetic nicotine, including some e-cigarettes and nicotine pouches, had a spurt of popularity due to a regulatory loophole that the FDA recently closed.
Why might patients who smoke consider a smokeless product?
Oral products can be used discreetly and hands-free. They create no smoke, vapor, or smell. The small size and clean appearance of tobacco-free pouches may make them a plausible alternative to cigarettes for women, or for any smoker with a “yuck” reaction to traditional moist snuff. There is no spitting or mess with pouched products.
E-cigarettes
Electronic cigarettes, also called vapes, are the best-known, and most-debated, category of nicotine alternative.[16]
What kinds of e-cigarettes are there?
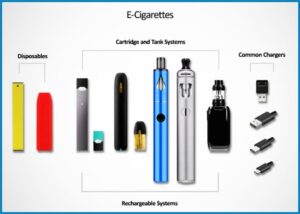
- Disposable e-cigarettes. One disposable vape may give hundreds of puffs, equal to a pack or two of cigarettes.
- “Closed system” devices. These use replaceable pods or cartridges.
- Open system” devices, with refillable tanks, can be used with any e-liquid.
At a glance, some e-cigarettes resemble traditional cigarettes. Others look more like pens or USB sticks. Tank devices are larger, and of varied and customizable appearance.
A CDC report[17] says 269 brands of e-cigarette were sold in U.S. stores and gas stations as of December 2022. The top sellers included prefilled cartridge products from Vuse, JUUL, and NJOY, and disposable vapes from Elf Bar and Breeze Smoke. Many more kinds may be sold online and in specialty shops.
As of mid-2023, the FDA had authorized the marketing of 23 tobacco-flavored e-cigarette products and devices. The FDA is still reviewing many more vaping products.
How risky is vaping compared to smoking?
Based on 78 studies, the Cochrane Collaborative finds high-certainty evidence that e-cigarettes work better than NRT to help people quit smoking.[18] Population-level trend modeling shows smoking rates dropping at a faster clip than expected, in ways correlated with the growth in vaping.[19]
A recent commentary in Nature Medicine[20] notes that “the number of chemicals in e-cigarette aerosol are orders of magnitude lower” versus cigarettes, and toxicants present in much lower concentrations. Some patients report pulmonary symptoms from vaping.
What about long-term risks? It’s hard to know, given that most vapers previously smoked, and their length of use is too brief to see outcomes such as COPD.
In a 2018/19 nationwide survey of physicians, over two-thirds (69.8%) said a patient has asked them about e-cigarettes. One in five (21.7%) had recommended them to a patient who smoked.6
Why might patients who smoke consider e-cigarettes?
Qualitative research with people who switched successfully from smoking says that vaping copies the “biopsychosocial” experience of smoking. That is, using an e-cigarette can feel and look like smoking.[21]
The variety of products can help patients find a match for their personal needs: from a simple “cigalike” product to a customizable high-tech one. And there’s the option to buy low- or zero-nicotine e-liquids. A patient switching from smoking to vaping could gradually reduce their nicotine level and then quit vaping. Or, they could continue vaping but quit nicotine.
Finally, flavors other than tobacco may attract adults from smoking to vaping. National studies, including a recent paper by FDA authors[22], have linked flavored e-cigarette use to greater success at quitting smoking.
Heated Tobacco Products
What on earth is a heated tobacco product?
Most physicians don’t know about[23] this third type of nicotine alternative: the heated tobacco product (HTP). It’s sometimes called heat-not-burn. Like e-cigarettes, HTPs are electronic devices. But they don’t vaporize a nicotine liquid. Instead, they heat tobacco-filled paper-wrapped sticks. This generates a nicotine-containing aerosol, which the user inhales. No burning or smoke means fewer toxins and carcinogens compared to a cigarette.

HTPs are rapidly transforming nicotine use in countries such as Japan. There, HTPs are the second-most-used product after cigarettes.[24] Internationally, brands such as IQOS, glo and Ploom are increasingly popular alternatives to smoking.[25] In the U.S., the FDA has authorized the marketing of IQOS. More HTPs are under review.
How risky is heated tobacco compared to smoking?
HTPs are newer and less-studied than e-cigarettes. Do people have lower exposure to some toxicants and carcinogens when they use HTPs instead of cigarettes? A Cochrane review[26] of randomized controlled trials found moderate-certainty evidence for that. But we need more studies to know whether HTPs help people quit smoking.
The FDA allows IQOS to carry this reduced-exposure message: “Scientific studies have shown that switching completely from conventional cigarettes to the IQOS system significantly reduces your body’s exposure to harmful or potentially harmful chemicals.”
Why might patients who smoke consider HTPs?
Like e-cigarettes, HTPs may help those who crave the familiar physical sensations of smoking. In a U.K. survey of nicotine users[27], common reasons for trying HTPs were curiosity; lack of smell, smoke and ashes; greater social acceptability; cutting down or quitting cigarette use; and enjoying the flavors or taste. Forty-three percent said a health professional had advised them to try HTPs.
The upshot: When to consider nicotine alternatives
Cigarettes are appealing, addictive, and toxic. NRT products are minimally harmful, but after twenty years of promotion, we have to admit that they “lack widespread appeal among smokers.”[28] We don’t have the evidence to prove that smokeless tobacco, e-cigarettes or heated tobacco products are as low-risk as NRT, but they are FAR less risky than cigarettes.
As Karl Fagerstrom4 notes, “Realistically, no single alternative nicotine product category will be able to reduce smoking rates and the associated disease burden.” Individuals and nations will find different alternatives appealing and acceptable.
The FDA recommends[29] educating adults who smoke about the relative risks of tobacco products. Encourage patients to try quitting with medication options. If that fails, one of the product types discussed here may save their lives.
Citations
[1] Kleykamp BA, Kulak JA. Cigarette use among older adults: A forgotten population. American Journal of Public Health. 2023;113(1):27-29. https://doi.org/10.2105/AJPH.2022.307151
[2] Van Eerd EAM, Risør MB, Spigt M et al. Why do physicians lack engagement with smoking cessation treatment in their COPD patients? A multinational qualitative study. NPJ Primary Care Respiratory Medicine. 2017;27:41. https://doi.org/10.1038/s41533-017-0038-6
[3] Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta‐analysis. Cochrane Database of Systematic Reviews. 2013. https://doi.org/10.1002/14651858.CD009329.pub2
[4] Fagerstrom K. Can alternative nicotine products put the final nail in the smoking coffin? Harm Reduction Journal. 2022;19:131. https://harmreductionjournal.biomedcentral.com/articles/10.1186/s12954-022-00722-5
[5] Hrywna M, Bover-Manderski MT, Wackowski OA, Steinberg MB, Delnevo CD. US physicians’ self-reported discussions about tobacco-free nicotine pouches during clinical encounters with patients in 2021. JAMA Network Open. 2023;6(5):e2313583. doi:10.1001/jamanetworkopen.2023.13583
[6] Delnevo CD, Jeong M, Teotia A et al. Communication between US physicians and patients regarding electronic cigarette use. JAMA Network Open. 2022; 5(4):e226692.
doi:10.1001/jamanetworkopen.2022.6692
[7] Warner KE. How to think, not feel, about tobacco harm reduction. Nicotine & Tobacco Research. 2019;21(10):1299-1309. https://academic.oup.com/ntr/article/21/10/1299/4990310
[8] Hecht SS, Hatsukami DK. Smokeless tobacco and cigarette smoking: Chemical mechanisms and cancer prevention. Nature Reviews Cancer. 2022;22(3):143-155. https://doi.org/10.1038%2Fs41568-021-00423-4
[9] Delnevo CD, Hrywna M, Miller Lo EJ, Wackowski OA. Examining market trends in smokeless tobacco sales in the United States: 2011-2019. Nicotine & Tobacco Research. 2021;23(8):1420-1424. doi:10.1093/ntr/ntaa239
[10] Inoue-Choi M, Shiels MS, McNeel TS et al. Contemporary associations of exclusive cigarette, cigar, pipe, and smokeless tobacco use with overall and cause-specific mortality in the United States. JNCI Cancer Spectrum. 2019;3(3):pkz036. doi:10.1093/jncics/pkz036
[11] Timberlake DS, Nikitin D, Johnson NJ, Altekruse SF. A longitudinal study of smokeless tobacco use and mortality in the United States. International Journal of Cancer. 2017;141:264-270. doi: 10.1002/ijc.30736
[12] Ramström L, Borland R, Wikmans T. Patterns of smoking and snus use in Sweden: Implications for public health. International Journal of Environmental Research and Public Health. 2016;13:1110. doi:10.3390/ijerph13111110
[13] Azzopardi D, Liu C, Murphy J. Chemical characterization of tobacco-free “modern” oral nicotine pouches and their position on the toxicant and risk continuums. Drug and Chemical Toxicology. 2021. https://doi.org/10.1080/01480545.2021.1925691
[14] Hrywna M, Gonsalves NJ, Delnevo CD, Wackowski OA. Nicotine pouch product awareness, interest and ever use among US adults who smoke, 2021. Tobacco Control. 2022. doi:10.1136/tobaccocontrol-2021-057156
[15] Jordt SE. Synthetic nicotine has arrived. Tobacco Control. 2023;32:e113-e117. doi:10.1136/ tobaccocontrol-2021-056626
[16] Balfour DJK, Benowita NL, Colby SM et al. Balancing consideration of the risks and benefits of e-cigarettes. American Journal of Public Health. 2021;111(9):1661-1672. https://doi.org/10.2105/AJPH.2021.306416
[17] Ali FRM, Seidenberg AB, Crane E et al. E-cigarette unit sales by product and flavor type, and top-selling brands, United States, 2020–2022. MMWR. 2023;72(25):672-677. http://dx.doi.org/10.15585/mmwr.mm7225a1
[18] Hartmann-Boyce J, Lindson N, Butler AR et al. Electronic cigarettes for smoking cessation. Cochrane Database of Systematic Reviews. 2022. https://doi.org/10.1002/14651858.CD010216.pub7
[19] Foxon F, Selya A, Gitchell J, Shiffman S. Population-level counterfactual trend modelling to examine the relationship between smoking prevalence and e-cigarette use among US adults. BMC Public Health. 2022;22:1940. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9580416/
[20] Warner KE, Benowitz NL, McNeill A, Rigotti NA. Nicotine e-cigarettes as a tool for smoking cessation. Nature Medicine. 2023;29:520-524. https://doi.org/10.1038/s41591-022-02201-7
[21] Notley C, Ward E, Dawkins L, Holland R. User pathways of e‐cigarette use to support long term tobacco smoking relapse prevention: a qualitative analysis. Addiction. 2020;116:596-605. doi:10.1111/add.15226
[22] Chang JT, Mayer M, Jackson RA et al. Characteristics and patterns of cigarette smoking and vaping by past-year smokers who reported using ENDS to help quit smoking in the past year: Findings from the 2018-2019 Tobacco Use Supplement to the Current Population Survey. Nicotine & Tobacco Research. 2023;25(3):596-601. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10215980/
[23] Wackowski OA, Steinberg MB, Delnevo CD. Impact of IQOS modified risk messaging on physicians’ product perceptions and recommendations. Tobacco Control. 2023. doi:10.1136/tc-2023-057963
[24] Odani S, Tabuchi T. Prevalence and denial of current tobacco product use: Combustible and heated tobacco products, Japan, 2022. Preventive Medicine Reports. 2022;30:102031. https://doi.org/10.1016/j.pmedr.2022.102031
[25] Sun T, Anandan A, Lim CCW et al. Global prevalence of heated tobacco product use, 2015–22: A systematic review and meta-analysis. Addiction. 2023;118:1430-1444. https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/add.16199
[26] Tattan-Birch H, Hartmann-Boyce J, Kock L et al. Heated tobacco products for smoking cessation and reducing smoking prevalence. Cochrane Database of Systematic Reviews. 2022. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD013790.pub2/pdf/full
[27] Brose LS, McDermott MS, McNeill A. Heated tobacco products and nicotine pouches: A survey of people with experience of smoking and/or vaping in the UK. International Journal of Environmental Research and Public Health. 2021;18:8852. https://doi.org/10.3390/ijerph18168852
[28] Abrams DB, Glasser AM, Pearson JL et al. Harm minimization and tobacco control: Reframing societal views of nicotine to rapidly save lives. Annual Review of Public Health. 2018;39:193-213. https://doi.org/10.1146/annurev-publhealth-040617-013849
29] King BA, Toll BA. Commentary on Wackowski et al.: Opportunities and considerations for addressing misperceptions about the relative risks of tobacco products among adult smokers. Addiction. 2023. https://doi.org/10.1111/add.16296COMMENTARY3



